10 sierpnia 2022 r. First use of the PENUMBRA Lightning 12 system in the Pulmonary Circulation Centre, John Paul II Hospital, Krakow, Poland
First use of the PENUMBRA Lightning 12 system in the Pulmonary Circulation Centre, John Paul II Hospital, Krakow, Poland
Jakub Stępniewski, Wojciech Magoń, Grzegorz Kopeć
61 y.o male. Caucasian. Previous history of arterial hypertension and overwight (BMI 29,98 kg/m2). No identifiable VTE risk factors. Signs of right leg DVT below knee 4 days prior to admission. Gradual decrease of exercise capacity for last 3 days. On the admission day sudden dyspnoea with syncope.
Admitted to a peripheral hospital Emergency room with normotension (SBP 110-120), tachycardia (HR 120-150), hypoxemia (SatO2 80% room air, rising to 85-88% on mask OXY).
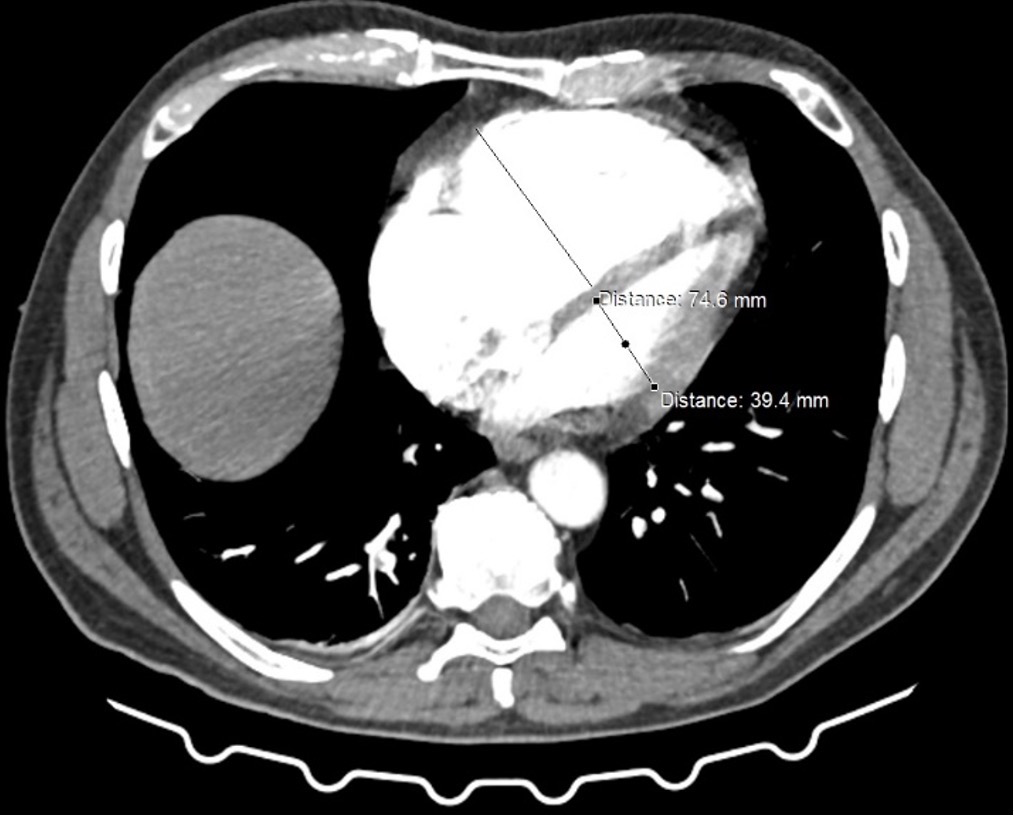
PA angioCT scan: bilateral, central clots. RV/LV ratio: 75/39=2,0
High sensitivity TroponinT levels: elevated [0,107ng/mL (N<0,014)]
Estimated early death risk by ESC criteria – intermediate-high risk.
Initial treatment: LMWH 80mg s.c
Transfered to our PERT center. No improvement of the clinical status, vital signs, and RV function during 12 hours of anticoagulation (LMWH changed to UFH dosed by aPTT); troponins increasing.
Consulted by the PERT (PE invasive specialist and cardiac surgeon) and qualified for catheter-directed treatment.
No contraindication to lysis.
Decision to use PENUMBRA Lightning 12 as a need for rapid RV debulk.
Access site: right femoral vein COOK 12F 80cm sheath
RHC (prior ; post)
– RA: 14 ; 13
– PA: 65/24/39 ; 44/21/29
Procedure:
Aspiration catheter: Indigo 12 XTORQ Tip 100cm Lightning apiration tubing
Separator: Indigo SEP12
Clot aspiration from:
– RPA intermediate artery, segemtal 10, 9, 8, 1+3
– LPA intermediate artery, segmental 9, 10
Radiation dose: 145mGy
Contrast media: 180mL
Estimated Blood Loss: ca. 350ml
No periprocedural or postprocedural complications.
Hb decrease: from 12 g/dL to 11 g/dL (min post-procedural level)
NT-proBNP decrease: from 14425 pg/mL to 10705 pg/mL (5h post procedure) and to 2520 pg/mL (48h post procedure)
Patient mobilized on the second post-procedural day. Discharged home on the 7th postprocedural day on rivaroxaban with no exertional dyspnea.
Figure. RV/LV ratio assessed on the antioCT at baseline (panel left) and post procedure (panel right)